How Do Gas Molecules Move Across a Room
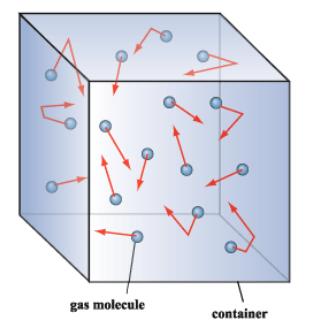
Unlike the molecules in a solid or in a liquid the molecules in a gas generally have a lot of empty space between them. In a gas particles have vibrational rotational and translational motion allowing them to bounce off of one another.
How Are Molecules Arranged In The Three States Of Matter Quora
Diffusion is the rate at which a gas travels across a room.

. When the barrier is removed and the molecules move into the vacuum they do it with different speeds given by the Maxwell distribution. Gas molecules moves because it is hot and it sperd apart. When not acted upon by a force a body either remains at rest or continues to move in a straight line.
4 It was discovered by Robeson in the early 1990s that polymers with a high selectivity have a low permeability and opposite is true. So at a low temperature the molecules have on the average less kinetic energy than they do at a high. Thus the molecules of gas move freely.
The temperature of a gas is also important in determining its molecular speed. Both of these phenomena are illustrated by the following figure. The molecule is also moving in the y and z axes so the answer depends on what exactly you mean by average speed.
Gas molecules can be packed into this space so gasses are compressible. Because the KMT states that the amount of kinetic energy is dependent on the temperature the temperature will determine how fast the molecules go in the first place. Effusion is the tendency of the particles of a gas to escape through a tiny hole in a.
Gases When you add even more energy to the substance you increase the kinetic energy of those particles so much that they lose their state form becoming a gas. This is an immediate consequence of the Newtons first law. Im voting to close this question as off-topic because its not exactly about chemistry.
If gas molecules are moving at a tremendous speed why does it require several minutes for a scent to diffuse across a room. To store gas molecules it is required to have a closed container. The particles are moving much faster than in a solid.
If a small sample of a very odorous and poisonous gas H2S is released in one corner of a room our noses will not detect it in another corner of the room for several minutes unless the air is vigorously stirred by a mechanical fan. But in any circumstance where you are in a room and concerned about odors propagating convection is the dominant way significant quantities of gases move around and mix up. Their molecular attraction is minimum when compared to liquid and solid molecules.
How would an increase in atmospheric pressure be expected to affect the rate of diffusion in a gas. Because a gas is a large number of very small particles moving very quickly and in random directions the gas will tend to spread out to all the space. These molecules flow in all directions and show a constant random and free molecular motion.
True diffusion is quite slow. Effusion is the rate at which a gas escapes through a small hole in a container. V x 298 m s 667 m p h.
There are five gas laws that affect the behavior of the gas and they are Boyles law Charless law Gay-Lussacs law Avogadros Law and Ideal gas law. Similar calculations may be performed for other substances. The slow diffusion of gas molecules which are moving very quickly occurs because the gas molecules travel only short distances in straight lines.
If the object passes at a low speed typically less than 200 mph the density of the fluid remains constant. But for high speeds some of the energy of the object goes into compressing the fluid moving the molecules closer together and changing the gas density which alters the amount of the resulting force on. Another process that involves movement of the particles of a gas is effusion.
4 the molecules are moving randomly in direction you are in a closed container with no wind or convection. The table below gives the Difference Between Liqu. The membrane is considered to have holes which the gas can dissolve solubility and the molecules can move from one cavity to the other diffusion.
Other important behaviors of gases explained by the Kinetic Molecular Theory are effusion and diffusion. Diffusion is the tendency of the particles of a gas to move from an area of high concentration to an area of low concentration. At a low temperature a gas molecule travels on the average at a slower speed than than it would at a high temperature.
So all that really matters is what the temperature of the gas is and what the gas is made of. Of course at a given temperature lighter molecules always move more quickly than heavier ones. Feb 18 2016.
Gas molecules have no definite volume and shape. T the temperature of the gas. How do gas molecules move.
Also check out How do gas molecules break newtons laws of motion and discussion therein. The molecules move all the time with a speed distribution dependent on the temperature of the gas. Gas molecules move around an object as it passes through.
Materials with a low selectivity have a high permeability. Explain how an increase in temperature would be expected to affect the rate of diffusion in a gas. This ignores rotational and vibrational degrees of freedom.
Behaviour of gas molecules depends on the temperature pressure volume and quantity of the gas molecules. But diffusion still does act and the molecules are buzzing around at quite fast spe. In a gas the molecules have a very weak intramolecular bond with each other due to the lack of kinetic energy in the bond.
The Temperature of the Gas. In other words diffusion is the tendency of the particles of a gas to spread out and fill a room.


Comments
Post a Comment